Pharmaceutical Services & Sciences
- 19057
- (202) 25351500, Ext.4165
- [email protected]

Latest Technologies
Compounding Isolator (SCI)
provides a sterile environment for aseptic preparations. It is factory-configured to operate at positive or negative pressure in a recirculating or total exhaust airflow scheme.
This compounding aseptic/sterile containment isolator is designed in compliance with international cGMP standards (Current Good Manufacturing Practice) and classified under Class 3 Leak Tight Containment as per ISO 10648-2.
Positive pressure is intended for handling of sterile non-hazardous materials while negative pressure is for containment of sterile hazardous substances.

Key Benefits:
- Complies with the international cGMP standards.
- Class 3 pressure tested as per ISO 10648-2.
- ISO Class 5 air cleanliness as per ISO 14644-1.
- Fast purging time.
- Compliant to USP <797> and <800>.
Automate Your Workflow with Breakthrough Sterile Drug Compounding Technology.
Introducing Diana, the first user-controlled automated compounding system for the safe preparation of IV medications, including hazardous drugs.
The Diana™ system helps you reduce the risk for exposure to hazardous drugs while maintaining drug sterility and minimizing physical stress to pharmacists and technicians.
Diana helps reduce human data entry errors and improve preparation traceability. Diana fits under the hood of a biological safety cabinet and uses many of ICU Medical’s proven ChemoClave® components to protect clinicians from exposure to hazardous drugs and accidental needle sticks, while protecting the final patient preparation from exposure to environmental contaminants.
Providing a Complete Record of Each Preparation:
After each mix is completed, the Diana system sends the medication name, volume, time, and date to a printer that creates up to five labels containing preparation information to complement your validation and quality assurance process, help prevent medication errors, and provide a record of past preparations.
BD Rowa™ Dose System

BD Rowa™ Dispensing Robotic provides us with a smooth smart safe efficient medication process
(24 hours a day, 7 days per week)
Dispensing Process for Oral Medications with BD Rowa™ Dose System at CCHE 57357:

- BD Rowa™ technologies streamline medication management: from ordering and logistics, to storage and dispensing to our patients.
- This allows healthcare professionals to free-up more time for patient care and guarantee a high level of medication safety at the same time.

Reinventing Medication Management Safer, Easier, and Smarter!
Optimizing Dispensing Process at CCHE 57357 through BD Rowa Dose System by:
- Eliminating picking errors
- Reducing LASA (Look-alike, Sound-alike) errors
- Elevating standards of provided patient service
- Increasing medication safety
- Managing medications expiry dates automatically
- Reducing manual steps in the storage of medications
- Reducing inventory errors
- Optimizing medication storage and stock flow at the hospital
- Fixed time within seconds without personal variance for collecting each medication
- Reducing patient waiting time


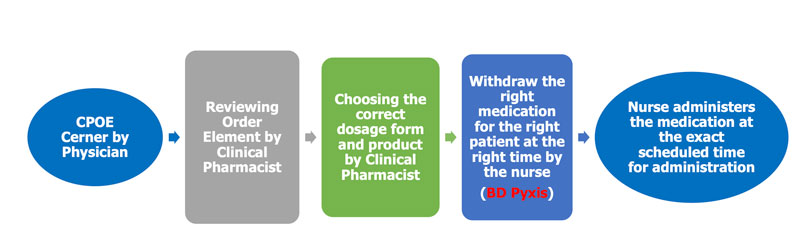
BD Pyxis Automated Unit Dose Dispensing Machine System
Description:
This dispensing machine is manufactured to decrease human errors to its max by confirming the right dose, right dosage form, right time and right drug by scanning its barcode.
The barcode is generated by Clinical Pharmacist while preparing the drug into unit forms and label it to identify: the dose, the concentration of each medication, batch number and expiration date.
Not only decreasing the human errors in dispensing or giving care to the patients, It’s also supply chain friendly cause it calculated the stock inside, the stock needed to be refilled and when (by defining the time of dispensing the drugs for patients or on hospital protocols). As well as its favored for quality team cause its really restricted in dispensing the medication and each one who want to dispense any type of drugs must enter by his user (despite the restricted form of medications needs witness with charge nurse), So it’s really easy to check which one who has discrepancy in stock.
It’s not only abut time and restrictions, lifesaving medications can be dispensed by override options to save the patient life in a controlled way as much as possible (including adrenaline, epinephrine, BDZ’s), it’s very useful in restricted form drugs and narcotics cause actually it record everything about the patient and the drug for his dosage and the nurse who dispensed.

Medication Dispensing Process through BD Pyxis Automated Unit Dose Dispensing Machine System at CCHE 57357:
Optimizing Dispensing Process at CCHE 57357 through BD Pyxis Automated Unit Dose Dispensing Machine System by:
- Decentralizing helps improving medications accessibility.
- Allowing a faster ordering process.
- Eliminating selection errors.
- Reducing LASA (Look-alike, Sound-alike) errors.
- Elevating standards of provided patient service.
- A mutual medication window between both pharmacists and nurses.
- Increasing medication safety.
- Real-time view of inventory levels.
- Optimizing medication storage and stock flow at the hospital.
- Printing access patient reports.
- Allowing a free time for nurses.
Personalized Medicine Management Unit (PMMU)
Therapeutic drug monitoring (TDM) tests
It provide quantitative results showing the correlation between the drug concentration in blood and a beneficial therapeutic effect. Therapeutic drug monitoring aids physicians to determinate the optimal drug dosage for patients by monitoring serum or plasma drug concentrations to ensure medication effectiveness and to avoid toxicity.
Siemens Viva-E
- Descriptions
- Application
The Viva-E System provides a complete menu of gold standard EMIT assays for fast analysis of, therapeutic drugs, immunosuppressants, and drugs of abuse. As well as sample validity testing on a single bench top analyzer. Viva –E System provide results in as little as 10 minutes. Also up to 130 EMIT tests per hour with 51 sample onboard capacity have12 EMIT methods onboard. It provides therapeutic drug monitoring for several medications like (Amikacin, Caffeine, Carbamazepine, Cyclosporine, Digoxin, Disopyramide, Ethosuximide, Gentamicin, Lidocaine, Methotrexate, Mycophenolic Acid, Phenobarbital, Phenytoin, Primidone, Procainamide, Quinidine, Sirolimus, Tacrolimus, Theophylline, Tobramycin, Valproic Acid, Vancomycin, Gabapentin, Lamotrigine, Levetiracetam, Methotrexate, Topiramate, Zonisamide). 
Viva-E system used in PMMU for TDM of (Methotrexate, Amikacin, Vancomycin, Voriconazole)
Indiko Clinical Chemistry Analyzer:
- Descriptions
- Application
- Sample Acceptance
- Blood samples in plain tubes or Serum samples
- Except for Cyclosporine: Blood samples in EDTA purple tubes
Indiko chemical analyzer, fits ideally to laboratory space limitations. Indiko is flexible, easy operation instrument. Also different sample types can be analyzed at the same time up to two hours. It is cost-effective solution with unique, low-volume cuvette technology, for samples (2 to 120 uL) and for reagents (2 to 240 uL) so low waste overall. It is used for therapeutic drug monitoring for several classes of medications like Antibiotics, Antiepileptic medications, and immunosuppressants, 
Indiko is used in PMMU for TDM of (Cyclosporine, Amikacin, Vancomycin, Digoxin, Valproic acid and Phenytoin)
High performance liquid chromatography (HPLC)
It is the premiere analytical technique used in many pharmaceutical applications including potency, purity, performance assays, pharmacokinetics, bioanalytical testing, purification, and Quality Control (QC).
The 1290 Infinity II LC System:
- Description
UHPLC provides a better resolution than traditional HPLC due to the shorter column length and smaller sub-2-micron particles in the columns, which allow more analytical work in less time with valuable, reliable and authentic data. As UHPLC systems have specialized pumps that can deal with the higher pressures. 
6470 Triple Quadrupole LC/MS:
- Description
LCMS is a combination of liquid chromatography and mass spectrometry. Both UHPLC and (LC-MS) can be used to identify and quantify pharmaceuticals, food compositions, and other bioactive molecules. 
Application:
- UHPLC have been used in PMMU since 2016 for therapeutic drug monitoring (TDM) for some antifungals such as (Voriconazole and Posaconazole).
- Moreover, quality control and quality assurance tests done to ensure the accurate concentration of the active ingredient in medications before their acceptance in our hospital.
- Also to assure the injected medications concentration of the intravenous preparations that prepared in CCHE- 57357 intravenous admixture room.
- Liquid chromatography–mass spectrometry (LC-MS) is another device for TDM of medications that not detected by immunoassay technique or UHPLC coupled with UV or florescence detector instruments as (Busulfan and 6-Mercaptopurine).
Sample Acceptance:
Sample acceptance according to the purpose. Please contact us for more information
The Applied Biosystems QuantStudio 12K Flex Real-Time PCR System
- Description
The QuantStudio™ 12K Flex system is a highly flexible, comprehensive real-time PCR platform. The QuantStudio 12K Flex Real-Time PCR System includes easy-to-change blocks that can accommodate OpenArray™ plates, TaqMan™ array cards, 384-well, 96-well, and FAST 96-well plates for maximum flexibility and consistency.

QuantStudio™ 12K Flex Accufill System:
- Description
The QuantStudio™ 12K Flex OpenArray™ AccuFill™ System is the automated system for loading DNA samples from 384-well plate into OpenArray™ chips precisely. After the OpenArray™ chips are loaded, they are ready for real-time PCR on a compatible Applied Biosystems™ real-time PCR instrument.

Application:
It has been used for wide broad range of applications, including gene expression, microRNA, small noncoding RNAs, SNP genotyping, protein analysis, protein thermal shift, copy number variation, and pathogen detection. This usually helps in drug discovery, pharmaceutical target confirmation, microRNA profiling, and agriculture molecular testing.
In Personalized medicine management unit (PMMU), QuantStudio™ 12K Flex system has been used for DNA quantification and genotyping of DNA for drug metabolizing enzymes as like:
- TPMT & NUDT15: (6-Mercaptopurine, Thioguanine, Azathioprine).
- DPYD: (5- Flurouracil, Capecitabine, Flucytosine, and Tegafur).
- RARG, SLC28A3, UGT1A6: ( Anthracycline: Doxorubicin& Daunorubicin ).
- CYP2C1: ( Voriconazole, Clopidogrel, Proton Pump Inhibitors (PPI) : (Omprazole, Pantoprazole, Lansoprazole, Dexlansoprazole), and Sertaraline, Citalopram , Escitalopram.
- CYP2C9: (Phenytoin, (NSAID): Celecoxib, Flurbiprofen, Ibuprofen, Lornoxicam, Meloxicam, Tenoxicam,Piroxicam.
- CYP3A4, CYP3A5: Tacrolimus .
Sample acceptance:
Sample Forms: Extracted DNA, or Whole blood sample tubes.
Note: Sample collection and storage techniques are greatly needed to be precisely applied for better integrity of DNA in the samples. S
- Overview
- Specialized Patient Care Department
- Inpatient Services Department
- Clinical Pharmacy Education Unit
- Production & Supply Chain Dep.
- Ambulatory Care Department
- Decision Support Department
- Meet Our Team
- Achievements
- Publications
- Latest Technologies
- Photo Gallery
- Video Library
- Join Our Team
- Training Request
- To Apply for Residency Program